H2so4 Naoh Balanced Equation
Balance the equation NaOH H2SO4 Na2SO4 H2O using the algebraic method. Therefore a balanced equation is given below.
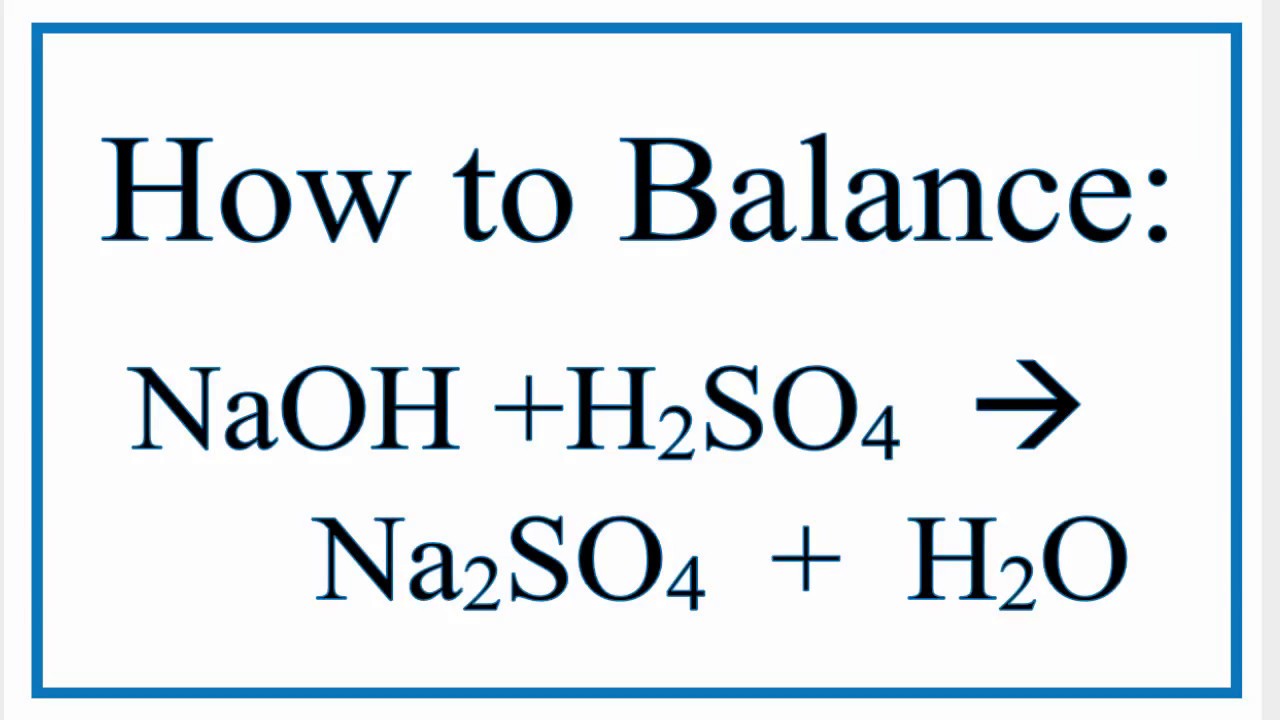
How To Balance Naoh H2so4 Na2so4 H2o Youtube
Example 2 Complex P 4 O 2 2P 2 O 5.

. H 2 SO 4 2NaOH Na 2 SO 4 2H 2 OBalanced Chemical Equation. Direct link to this balanced equation. 2NaOH 2H2SO4 Na2SO4 2H2O.
H2SO4 2NaOH - 2H2O Na2SO4 We are told that 20. Na 2 SO 4. By using the ionic equation we can find out the spectator ions by splitting each.
This equation is a balanced equation because there is an equal number of atoms of each element on the left and right hand sides of the equation. There are three main steps for writing the net ionic equation for H2SO4 NaOH Na2SO4 H2O Sulfuric acid Sodium hydroxide. Of atoms in reactantsNo.
In a neutralization reaction with a strong acid or base all of the hydrogens from the acid and the hydroxides from the base react to form water. Please tell about this free chemistry software to your friends. Balance the following neutralization reaction.
Label Each Compound With a Variable. Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equationKNO 3FeSO 4H 2SO 4K 2SO 4Fe 2SO 4. H2SO4 reacts with NaOH.
To balance NaOH H2SO4 Na2SO4 H2O youll need to watch out for two things. Balanced Chemical Equation Solution. NaOH reacts with H2SO4.
A chemical reaction is said to be balanced when the total number of the atoms present in the reactant as well as product side are equal. ΣΔH f reactants ΣΔH f products so Na H2O NaOH H2 is exothermic. First be sure to count all of H Na S and O atoms on each side of the che.
Is H2SO4 2NaOH Na2SO4 2H2O. Naoh h2so4 net ionic equation. The limiting reagent row will be highlighted in pink.
Phenomenon after H2SO4 sulfuric acid reacts with NaOH sodium hydroxide This equation does not have any specific information about phenomenon. The coefficients show the number of particles atoms or molecules and the indices show. H 2 SO 4 2 NaOH Na 2 SO 4 2 H 2 O.
First we balance the molec. Molar mass - gmol weight - g. In a full sentence you can also say H2SO4 sulfuric acid reacts with NaOH sodium hydroxide and produce H2O water and NaHSO4 Phenomenon after H2SO4.
Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. In a full sentence you can also say H2SO4 sulfuric acid reacts with NaOH sodium hydroxide and produce H2O water and Na2SO4 sodium sulfate. Label each compound reactant or product in the equation with a.
Equation H2So4aqNaOHaqNa2So4aqH2Ol is an impossible reaction Instructions and examples below may help to solve this problem You can always ask for help in the forum. Balance the following chemical equationsb NaOH H2SO4 Na2SO4 H2OAnswerNaOH H2SO4 Na2SO4 H2OName of the elementNo. NaOH H 2 SO 4 Na 2 SO 4 H 2 O Balance it.
NaOH reacts with H2SO4.
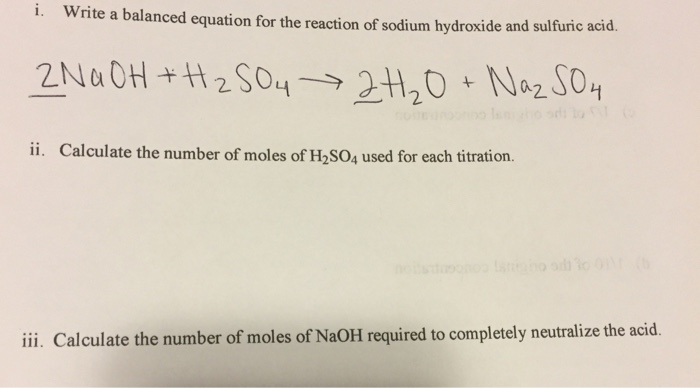
Solved Write A Balanced Equation For The Reaction Of Sodium Chegg Com

Type Of Reaction For H2so4 Naoh Na2so4 H2o Youtube

Sodium Hydroxide Sulfuric Acid Acid Base Neutralization Reaction Youtube
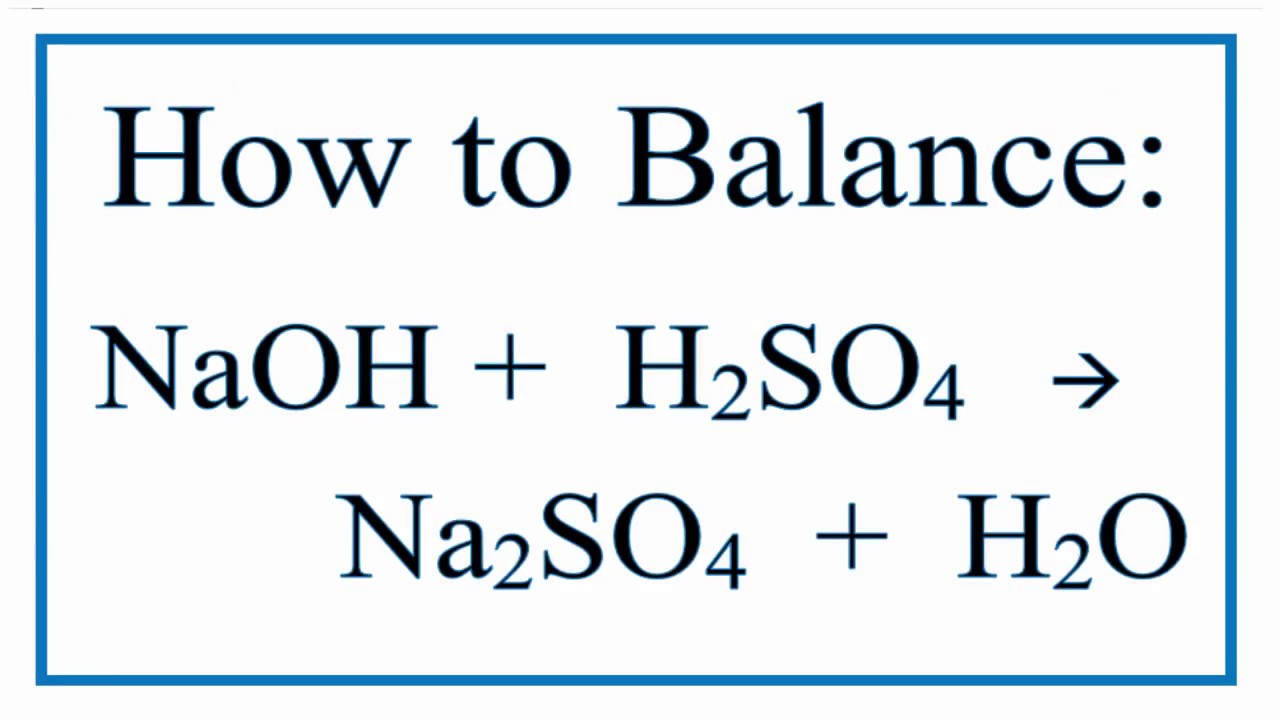
Sodium Hydroxide And Sulfuric Acid Yields Sodium Sulfate And Water Youtube

How To Balance Naoh H2so4 Na2so4 H2o Youtube
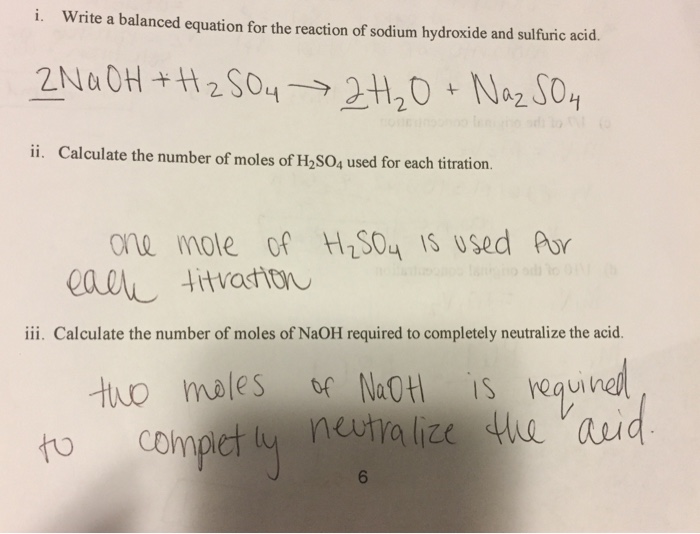
Solved Write A Balanced Equation For The Reaction Of Sodium Chegg Com
Comments
Post a Comment